Transition extending life of mine production underway
-
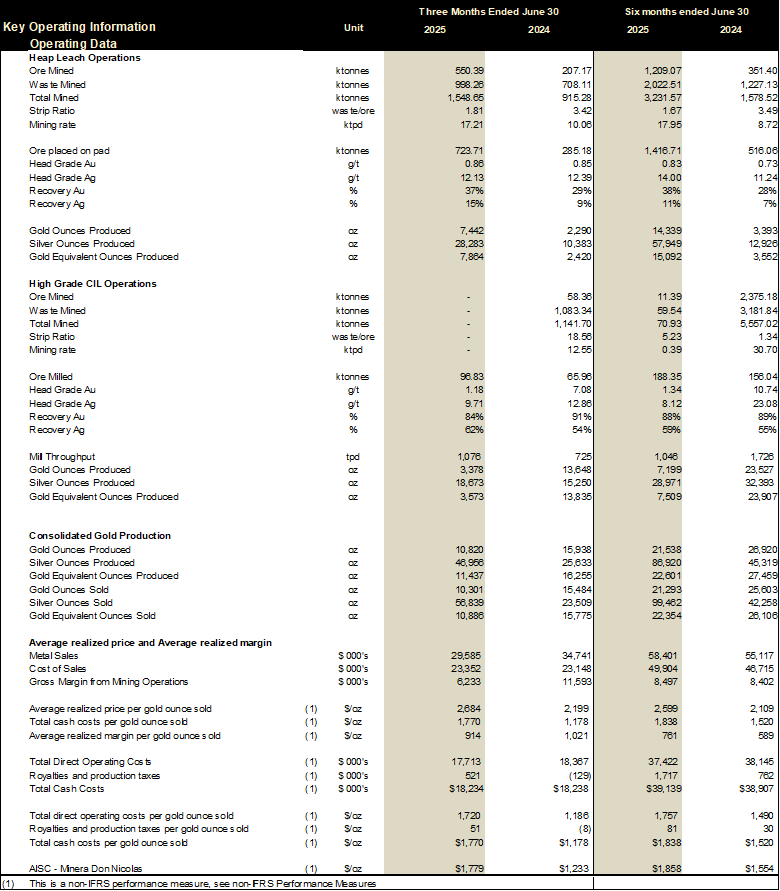
Gold equivalent production of 11,437 Gold Equivalent Ounces (“GEO”) at AISC of $1,779/oz during Q2 2025
-
Adjusted EBITDA of $7.4 million for Q2 2025
-
Full year guidance of 55,000-60,000 GEO maintained: Production weighted to H2 as higher-grade underground mining and heap leach volumes increase
-
Repayment of ~US$18m debt and payables at the MDN operations year to date- ~US$10m in Q2 2025
-
20,000 meter resource expansion exploration program at MDN underway
-
Significant investment and progress at both the Lagoa Salgada and Mont Sorcier projects
-
Positive Metallurgical results at Lagoa Salgada to be incorporated into Optimized Feasibility Study to be delivered by year end
-
Management to host conference call on Monday, 25th of August, 11AM EDT
TORONTO, ON / ACCESS Newswire / August 21, 2025 / Cerrado Gold Inc. (TSXV:CERT)(OTCQX:CRDOF)(FRA:BAI0) (“Cerrado” or the “Company“) announces its operational and financial results for the second quarter (“Q2/25”) including its Minera Don Nicolas (“MDN“) gold mine in Santa Cruz Province, Argentina, its Lagoa Salgada Polymetallic Project in Portugal and its Mont Sorcier High Purity DRI Iron Project in Quebec. Financial results now include the consolidated financial position of Ascendant Resources Inc. (“Ascendant”) following the close of the acquisition effective May 16, 2025.
Production results for MDN were previously released on July 17, 2025. The Company’s financial results are reported and available on SEDAR+ (www.sedarplus.com) and the Company’s website (www.cerradogold.com).
Q2/25 MDN Operating Highlights:
-
Q2/25 production of 11,437 GEO and AISC of $1,779/oz
-
Unit costs expected to continue to decline as production increases in H2/2025
-
-
Q2/25 Adjusted EBITDA of $7.4 million
-
Record heap leach production of 7,864 GEO during the Quarter
-
Underground development at Paloma started with three access portals
-
CIL plant receiving initial contribution from underground development; production expected to ramp up over H2/2025
Operational results for the second quarter saw gold production increase compared to Q1 2025, but were lower than Q2/24 during which period the high grade Calandrias Norte pit was being mined. This was anticipated given the depletion of high-grade material from Calandrias Norte, following which, MDN became primarily a heap leach only operation pending the ramp up of new high-grade material from underground. The heap leach operation reached another production record of 7,864 GEO for the quarter. The recently expanded crushing circuit is enabling higher volumes of ore to be placed on the pad, with further increases expected following the planned addition of an agglomerator and additional conveyors in Q3 2025. With higher gold prices, the CIL plant continued to process lower-grade stockpiles through Q2/25. Going forward, lower grade material will be blended with new high-grade material from underground mining feed beginning in Q3/25 which will increase mill grades, boost production and lower unit operating costs further.
Mark Brennan, CEO and Chairman commented, “The results from the second quarter demonstrate a robust operation transitioning to deliver increased production in the second half of the year and beyond. The expanded and improved crushing capacity at the heap leach is delivering greater production and when combined with new, higher-grade ore expected from underground operations, unit costs are expected to decline significantly in the second half of the year, enhancing our financial performance.”
He continued, “The strong cash flow generated from operations combined with our cash balance, has enabled us to pay down $10m in debt at MDN during the second quarter, while continuing to deploy capital for surface exploration and development of new resources underground. We are also seeing very positive advances at our Lagoa Salgada Polymetallic Project and at the High-grade Mont Sorcier DRI Iron Project in which we expect to unlock significant value that we see in these projects as the respective Feasibility Studies are completed.”
Operating Results for the Quarter
Operational results for Q2 2025 showed a modest increase in production over the previous quarter, driven by modestly higher production from the heap leach operations. The heap leach performance remained steady over the quarter. However, recovery rates were impacted by a larger amount of primary ore placed on pad during the quarter due to mine sequencing. This primary material has lower recovery rates and longer retention times as compared to oxide material. Heap leach production is expected to improve in H2/25 as more oxide ore is mined, the addition of an agglomerator to reduce fines is implemented, and the ongoing upgrades to the crushing circuit are completed. In addition, early in the quarter the crushing circuit was offline for approximately 15 days as upgrades were put in place to support higher throughput rates moving forward. Production from stockpiled material via the CIL plant declined somewhat due to lower grades, however, underground operations at Paloma are expected to begin to contribute meaningfully to production in H2 2025 and beyond as development rates increase and more ore becomes available.
The second phase of the expansion of the heap leach crushing circuit is now complete, which will increase feed stability in order to deliver steady ore volumes to the pad. While supporting higher production rates, additional crushing facilities are also expected to reduce the feed size to the pad and result in increased recoveries. The final updates to the crusher circuit, including final installation of the agglomerator and additional conveyors, are set to be completed in Q3/25.
As previously announced, MDN commenced underground mining in June, opening up three portals for underground mining beneath the Paloma pit. Ore production is expected to ramp up over H2 and is set to make a material contribution to production rates as the year progresses. While initial production expectations are relatively modest given the current known underground resource, underground access is expected to provide a platform for major exploration activities at a lower cost than drilling from surface. Underground exploration aims to materially expand resources at MDN, leveraging the underground development for a potential expansion in production and/or mine life.
On the exploration front, the Company commenced an approximate 20,000 metre drill program at MDN late in the quarter and is set to drill numerous targets in the coming months with the aim to potentially define new resources to provide mill feed to the CIL plant. Drilling commenced with a single DDH rig north of the Paloma pit, where several new veins have been intersected. Results are pending and further drilling will be required to confirm new resources.
In the near term, the focus at MDN remains to ramp up production rates at its heap leach operation to approximately 4,000-4,500 GEO per month, grow underground production from the Paloma area in H2, and ramp up a new targeted exploration program across our 330k Ha property to increase resources and mine life.
The Company has reduced its debt position by approximately $10M over the quarter at MDN and is well positioned to have a robust financial position by year end. Strong cashflow and reduced liabilities at MDN will support continued development and exploration at MDN, while pushing forward its strategic development programs at its projects in Quebec and Portugal.
Q1 Financial Performance
Table 1. Q1 2025 Operational and Financial Performance
The Company produced 11,437 gold equivalent ounces (“GEO”) during the three months ended June 30, 2025, as compared to 16,255 GEO for the three months ended June 30, 2024. In the period ended June 30, 2025, heap leach production was significantly higher compared to the prior year due to 27% higher recoveries and 438,530 additional tonnes placed on the pad. This was offset by a 10,262 ounce decrease in production from the CIL operation as the Company’s focus moved towards heap leach operations in 2025 and only processed low grade ore in Q2 2025.
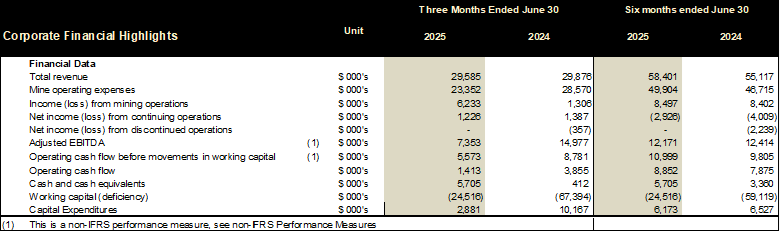
The Company generated revenue of $29.6 million for the three months ended June 30, 2025, from the sale of 10,301 ounces of gold and 56,839 ounces of silver at an average realized price per gold ounce sold of $2,684. For the three months ended June 30, 2024, the Company generated revenue of $34.7 million from the sale of 15,484 ounces of gold 23,509 ounces of silver. Revenue is lower for the three months ended June 30, 2025 as compared to the three months ended June 30, 2024, due primarily to lower gold ounces sold.
Cost of sales for the three months ended June 30, 2025, were $23.4 million as compared to $23.1 million for the three months ended June 30, 2024. The Company incurred $0.6 million higher production costs for the three months ended June 30, 2025 due to slightly higher costs of operational contractors and labour costs in 2025 as compared to 2024. Sales expenses were also slightly higher in the first quarter of 2025 by $0.6 million, offset by a $1.0 million decrease in depreciation expense.
Total cash costs (including royalties) sold was $1,770 per ounce in the three months ended June 30, 2025, as compared to $1,178 per ounce for the three months ended June 30, 2024, a $592 per ounce or 50% increase (refer to reconciliation of Non-IFRS performance metrics). The increase is a result of lower ounces sold in 2025 as compared to 2024.
Net income from continued and discontinued operations for the three months ended June 30, 2025, was $1.2 million as compared to a net income of $1.0 million for the three months ended June 30, 2024. The increase in net income is primarily a result of decrease in foreign exchange loss of $5.9 million and lower depreciation expense of $1.0 million offset by $5.2 million decrease in metal sales, an increase in loss on remeasurement of MDN stream obligation of $0.8 million and an increase in loss on remeasurement of Ascendant secured note and stream obligation of $1.1 million.
The Company incurred general and administrative expenses of $2.8 million for the three months ended June 30, 2025, as compared to $2.5 million of general and administrative expenses incurred during the three months ended June 30, 2024. The increase was primarily a result of an increase in salaries of $0.1 million, consulting fees of $0.1 million and marketing and promotion of $0.1 million offset by a decrease in stock-based compensation of $0.1 million, and office and other of $0.1 million for the three months ended June 30, 2025.
Other loss of $1.4 million during the three months ended June 30, 2025, includes finance expense of $1.4 million, loss on fair value remeasurement of MDN stream obligation of $1.0 million and loss on fair value remeasurement of Ascendant secured note and stream obligation of $1.1 million offset by finance income of $1.0 million and foreign exchange gain of $0.9 million.
Hedging Program
On April 26, 2025, the Company extended its limited hedging program with Ocean Partners UK Ltd. The hedge is constructed as a zero-cost collar with lower and upper boundaries of US$3,100 and US$3,250 per ounce respectively. The hedging volume is for 2,000 ounces per month for a period of 7 months beginning May 2025 and terminating on or about December 2025, subject to production volume. With the expanded hedging program, the Company is focused on ensuring more than sufficient cash flows to further enhance its balance sheet and support funding requirements for its various growth programs.
Outlook
Entering H2 2025 and beyond, Cerrado’s MDN Heap Leach operations are benefiting from the recent improvements to its crushing infrastructure to grow and improve production rates. Higher gold prices have enabled the CIL plant to remain operational by processing lower grade stockpiles, and it is now set to benefit from the introduction of higher-grade ore from underground operations, which is expected to improve overall profitability and free cash flows.
The Company has maintained its 2025 annual production guidance to 55,000 – 60,000 GEO. AISC costs are expected decline in the second half of 2025 as production ramps up to deliver AISC of between $1,500 – $1,700 per GEO.
The 20,000-meter exploration program has begun and is focused on growing the known resources at MDN beyond those outlined in the most recent Mineral Resource Estimate (“MRE”). The focus remains on defining high grade-near surface targets that can readily be brought into the mine plan. The Company has developed an underground and a regional program to better understand the potential of known anomalies on the significant land package Cerrado holds at MDN. Drilling is now underway.
During Q2 2025 the Company closed the acquisition of all of the outstanding common shares of Ascendant Resources Inc. not already owned by the Company and thereby obtaining an effective 80% interest in the Lagoa Salgada VMS project on the Iberian Pyrite Belt in Portugal. Accordingly, financial statements reflect the consolidation of Ascendant financial information for the first time, which includes liabilities assumed by the Company in connection with the acquisition.
At the Lagoa Salgada project, work continued across key workstreams with the goal of reaching a construction decision by Q1 2026. Ongoing metallurgical testing has already delivered positive improvements and is expected to be completed imminently. Management considers the previously reported improvements to Capex and Opex at Lagoa to be very encouraging, and they will be integrated into the optimization program for the OFS. Significant positive results using Dense Media Separation on the Stockworks zone has warranted further analysis on mine and plant design. The expected result is a reduction in processing costs from this domain, but requires further detailed work to be undertaken before it can be incorporated into the OFS. Parallel workstreams to complete the OFS are currently ongoing and it is expected that the completion of the OFS will occur by year end.
The Company is also advancing the approval in the Environment Impact Assessment (EIA), after successfully receiving Article 16 approval from the Portuguese regulators. The Company expects to submit its revised EIA in October.
At the Mont Sorcier high grade and high purity DRI iron project operated by Cerrado’s wholly owned subsidiary Voyager Metals Inc., work continued to advance the project with several workstreams related to permitting, social license and the initiation of the Feasibility Study which is targeted to be completed during Q1 2026. The high quality of the concentrate, grading over 67% iron, from the Mont Sorcier project is well positioned to support growing demand from the global Green Steel transition due to the reduced emissions generated by steel producers using high grade concentrates.
Conference Call Details
Cerrado Management will host a conference call on August 25, 2025, at 11:00 AM EDT to discuss the Q2 Financial and Operational results. The presentation for the call can be found on the investor page on Cerrado Gold’s website at cerradogold.com. Call details are as follows:
Pre-Registration for Conference Call
Participants can preregister for the conference by navigating to:
https://dpregister.com/sreg/10202340/ffc98dcb64
Participants will receive dial-in numbers to connect directly upon registration completion.
Those without internet access or unable to pre-register may dial in by calling:
PARTICIPANT DIAL IN (TOLL FREE): 1-833-752-3576
PARTICIPANT INTERNATIONAL DIAL IN: 1-647-846-8340
PARTICIPANT INTERNATIONAL DIAL IN: 1-647-846-8340
Review of Technical Information
The scientific and technical information in this press release has been reviewed and approved by Andrew Croal P.Eng, Chief Technical Officer for Cerrado Gold, who is a Qualified Person as defined in National Instrument 43-101.
About Cerrado
Cerrado Gold is a Toronto-based gold production, development, and exploration company. The Company is the 100% owner of the producing Minera Don Nicolás and Las Calandrias mine in Santa Cruz province, Argentina. In Portugal, the Company holds an 80% interest in the highly prospective Lagoa Salgada VMS project through its position in Redcorp – Empreendimentos Mineiros, Lda. In Canada, Cerrado Gold is developing its 100% owned Mont Sorcier Iron project located outside of Chibougamou, Quebec.
In Argentina, Cerrado is maximizing asset value at its Minera Don Nicolas operation through continued operational optimization and is growing production through its operations at the Las Calandrias heap leach project. An extensive campaign of exploration is ongoing to further unlock potential resources in our highly prospective land package in the heart of the Deseado Masiff.
In Portugal, Cerrado focused on the exploration and development of the highly prospective Lagoa Salgada VMS project located on the prolific Iberian Pyrite Belt in Portugal. The Lagoa Salgada project is a high-grade polymetallic project, demonstrating a typical mineralization endowment of zinc, copper, lead, tin, silver, and gold. Extensive exploration upside potential lies both near deposit and at prospective step-out targets across the large 7,209-hectare property concession. Located just 80km from Lisbon and surrounded by exceptional infrastructure, Lagoa Salgada offers a low-cost entry to a significant exploration and development opportunity, already showing its mineable scale and cashflow generation potential.
In Canada, Cerrado holds a 100% interest in the Mont Sorcier Iron project, which has the potential to produce a premium iron concentrate over a long mine life at low operating costs and low capital intensity. Furthermore, its high grade and high purity product facilitates the migration of steel producers from blast furnaces to electric arc furnaces, contributing to the decarbonization of the industry and the achievement of sustainable development goals.
For more information about Cerrado please visit our website at: www.cerradogold.com.
Mark Brennan
CEO and Chairman
Mike McAllister
Vice President, Investor Relations
Tel: +1-647-805-5662
mmcallister@cerradogold.com
Disclaimer
NEITHER TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
This press release contains statements that constitute “forward-looking information” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that discusses predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements.
Forward-looking statements contained in this press release include, without limitation, statements regarding the business and operations of Cerrado, anticipated continued improvements in operating results and working capital position, future production and grade estimates, future cashflows, expectations regarding the CIL plant processing lower grade stockpiles, the potential for improvement at MDN’s heap leach operation and higher grade ore being processed at MDN, expectations regarding improvements in operating costs at MDN including reduction in AISC, additional capacity being added at the heap leach operation, the potential of underground operation at MDN, the anticipated timing of completing the feasibility study at the Mont Sorcier project, the potential for a construction decision at Lagoa Salgada and the expected timing and likelihood of receiving approval of the environmental impact assessment at Lagoa Salgada. In making the forward- looking statements contained in this press release, Cerrado has made certain assumptions. Although Cerrado believes that the expectations reflected in forward-looking statements are reasonable, it can give no assurance that the expectations of any forward-looking statements will prove to be correct. Known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such factors include, but are not limited to general business, economic, competitive, political and social uncertainties. Accordingly, readers should not place undue reliance on the forward-looking statements and information contained in this press release. Except as required by law, Cerrado disclaims any intention and assumes no obligation to update or revise any forward-looking statements to reflect actual results, whether as a result of new information, future events, changes in assumptions, changes in factors affecting such forward-looking statements or otherwise.
SOURCE: Cerrado Gold Inc.
View the original press release on ACCESS Newswire
The post Cerrado Gold Announces Second Quarter 2025 Financial Results appeared first on DA80 Hub.